DFM Packaging Solutions: Saving Billions for More Than 40 Years
The medical packaging industry is unique—it requires extreme attention to detail and the ability to think outside the norm when designing. Yet, because packaging engineers are well aware of the hurdles that must be cleared to reach the market, it is also easy to gloss over innovative opportunities and stick with what has worked in the past.
This is a natural mindset. Engineers must solve issues in advance to ensure that packaging designs will pass human factor, transport, and validation testing, while protecting the device. Packaging requirements can also contradict each other, such as senior-friendly vs. child-resistant packaging, requiring further creativity. Through it all, timelines, supply chain, and costs loom.
In the early 70s, Geoffrey Boothroyd, a University of Massachusetts professor, became interested in devising a process to help design engineers consistently reduce product assembly cost. The principles he created were called Design for Assembly (DFA). Design for Assembly methods revealed the next phase of need, so he kept going, identifying ways to reduce manufacturing complexity and lower overall part production cost. This became Design for Manufacture, or (DFM). Those principles went on to become software-driven tools in the 80s and we haven’t looked back since. Today, DFA and DFM methodologies are considered two sides of the same coin, commonly referred to as DFMA.
We recently sat down with Mark Foster, General Manager at Oliver Design, to learn some of the most common DFMA advantages, benefits, and challenges. In the medical packaging world, the principles of Design for Manufacture are critical.
What do you mean when you say design for manufacture?
Design for Manufacture is exactly what it sounds like—designing with the manufacturing process in mind. This ensures your sketch can come to life and be produced by standard, scalable manufacturing processes. DFMA also optimizes efficiency and, as a result, saves time and money.
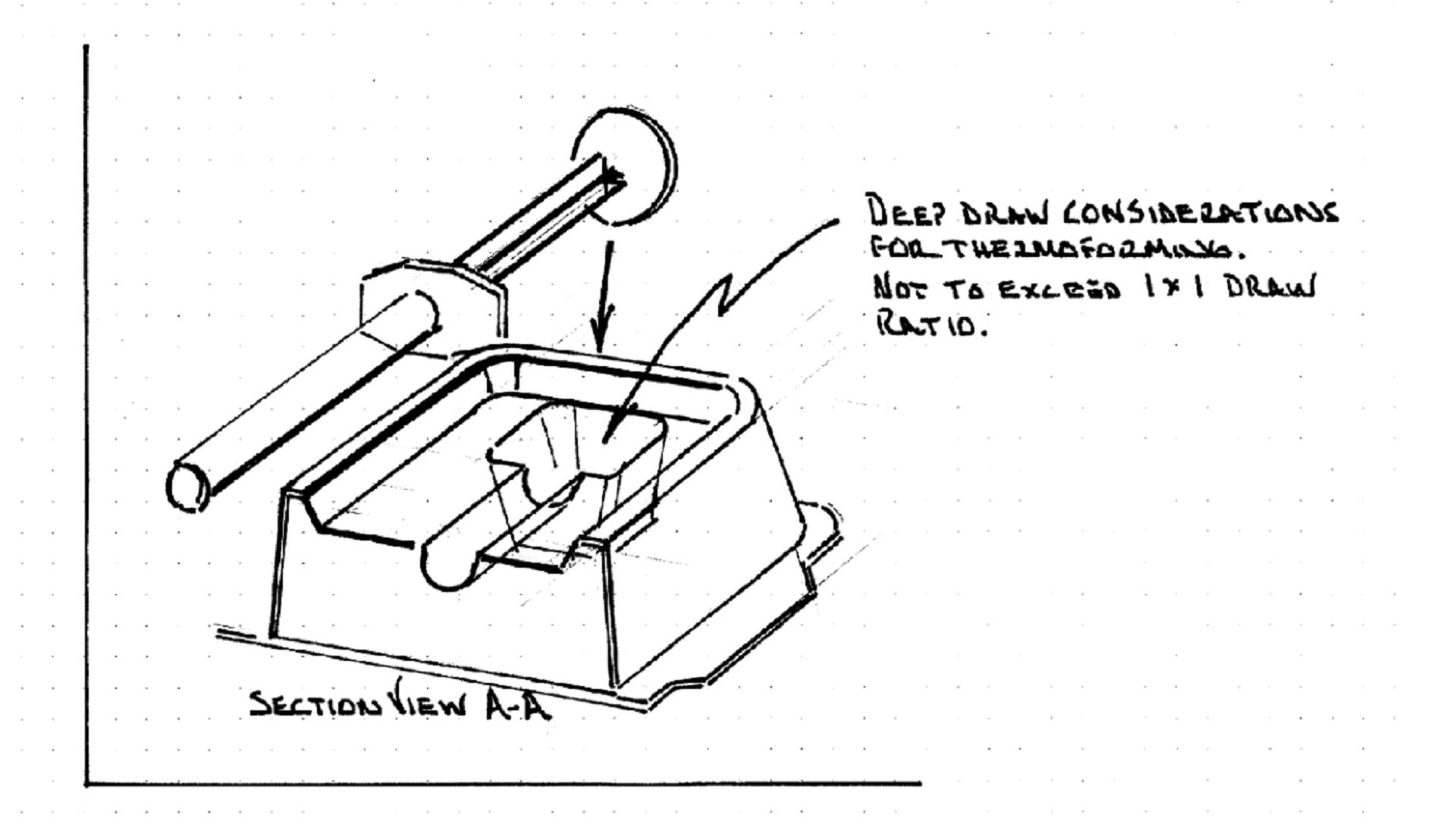
Can you provide an example of something that might be designed but can’t be manufactured?
Absolutely. Many times, side-by-side, the sketches might not look all that different. What it comes down to is the knowledge of what a manufacturer can and cannot do. Other times, the design might appear new and innovative but is missing key elements that make the packaging possible to produce, while still being protective. A good example is thermoformed trays and what we call deep-draw considerations. A tray’s depth should not exceed its width. When this rule is not followed, it can make manufacturing a tray very difficult or impossible. Another good example is discerning between when part designs require male or female molds. Working with a design team that understands production equipment constraints will avoid re-work downstream.
What is a common challenge you see from clients?
When our clients come to us, we see a wide array of challenges. However, one of the most common challenges we see, and one that can be avoided, is the scenario I mentioned above: packages that have been designed but can’t actually be manufactured. This can cause major delays in getting to market and is one of the reasons why Oliver Design puts such a focus on Design for Manufacture.
What are some of the advantages and benefits of applying DFMA?
We’re all constantly looking to reduce costs and add value. Those goals are the organic result when utilizing DFMA. Design for Manufacture reduces the number of parts to the lowest possible count while maximizing quality. This, in turn, reduces how many assembly steps are required, distilling the manufacturing run to its most efficient state, early in the game. While every medical device packaging system is a one-of-a-kind, Design for Manufacture breaks down barriers between concept and finished goods. The cost and time savings are enormous. There is no downside, and that’s another reason Oliver Design is all-in when it comes to deploying DFMA from the moment a project is out of the gate.