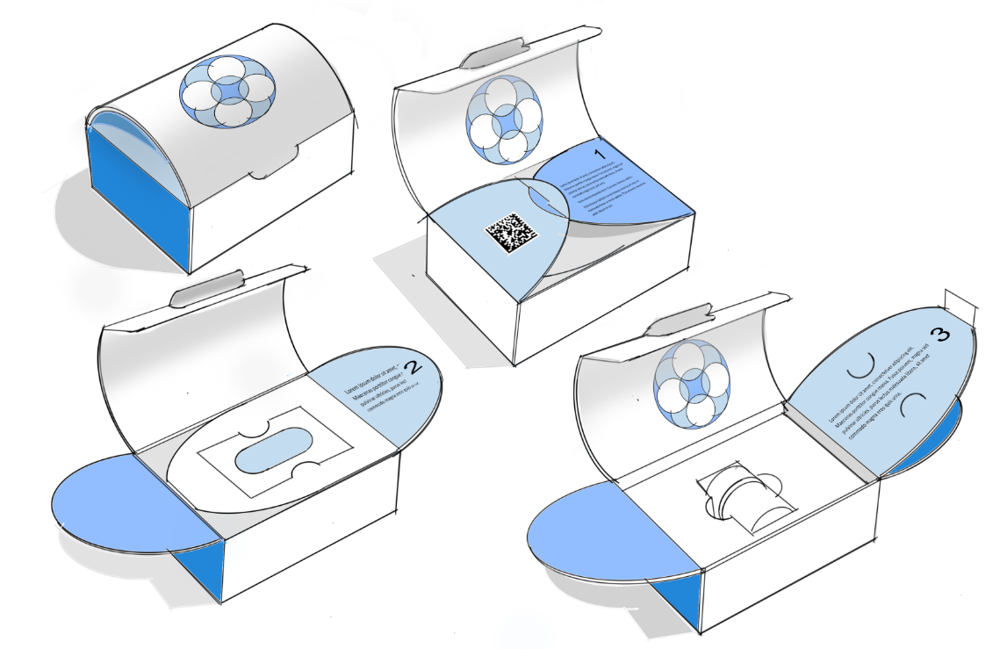
How to Ensure Secondary Packaging Success
The days of secondary packaging taking a back seat during drug development are changing, if not over. The ongoing rise of home administered therapies, particularly for parenteral drugs (intravenous, intramuscular, subcutaneous) turns secondary packaging from a protective layer for clinicians to a protective and instructional “step” of the total medicine delivery process. In short, drug makers, packaging engineers and manufacturers must understand what’s changed and how to meet the new requirements of secondary packaging.
Existing and new considerations and changes affecting parenteral therapies packaging (and delivery methodologies) are explored in this recent article. With so much under way, what can your parenteral therapy team do to stay ahead of this paradigm shift?
We suggest three action steps to start and finish a secondary packaging system to be proud of:
Action Step 1: Specialized Support
By specialized support, we mean expert—this is not the place to experiment. When concepting secondary packaging, such as a kit for patient or caregiver use, look for a partner with experiencein parenteral packaging and at-home administration. Your product will be well served by a specialized design firm that is solely dedicated to the medical industry. Working with designers who create yogurt cups or baby toy packaging one day, and then are ready and willing to help you with your PFS or auto Injector kit the next can seem innocuous, but more likely sets your path on a downhill slide.
Action Step 2: Design for Manufacturing
A critical question to ask is “Can we make this?” Many engineering concepts look amazing on paper and in principle. But when it comes to fabricating that concept, all hell can break loose. An effective packaging design effort must include design for manufacturing as a guiding principle. A lot of frustration haunts biopharma companies that work with design firms that offer really cool aesthetic solutions – until it becomes obvious that they couldn’t be manufactured at the scale required for the application. The ideas might be inspiring – but they’re not meant for production. Finding the balance will win at the end of the day.
Action Step 3: Build in Time and Human Factors
The mantra “Don’t procrastinate on packaging” exists for many reasons, primarily because it is painfully accurate. Do yourself and your company’s bottom line a favor and leave ample time to get this work done. Not only must the secondary packaging system accomplish its medical, sterility and protective requirements, it must be user-friendly, condition-specific, and “foolproof” to patients and families in sometimes stressful or rotating care scenarios at home. The best behavioral specialists will say “I don’t know” when asked how patients with specific challenges will interpret written instructions. Human factors studies yield surprising outcomes and insights. Tremendous learning comes from those studies and ample time should be allocated to completing that work.
Primary packaging comes with enough challenges of its own. The next generation of secondary packaging coming online adds twists we wouldn’t have dreamed of just a decade ago but are coming true today. Avoid nightmares, by discussing these three focus areas right now—wherever you are on the parenteral therapy journey. Better late than never still applies.